RFID in Medical Devices
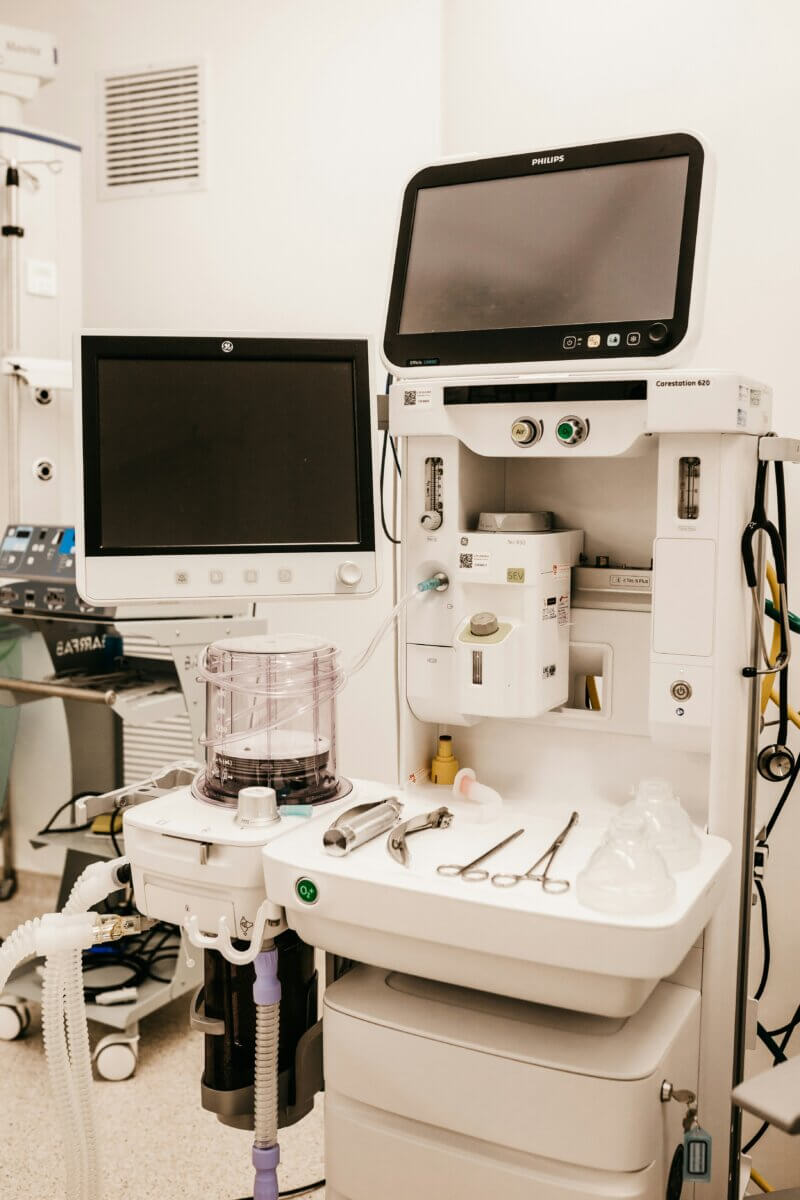
When a dialysis machine accepts the wrong filter, or an infusion pump fails to detect a counterfeit medication set, patient safety is at stake. That’s why more and more medical device manufacturers are integrating RFID directly into their equipment.
The Problem
Medical devices rely on consumables: infusion bags, dialysis filters, breathing circuits, contrast media cartridges. Each use requires the right material – in the correct configuration, within its expiration date, from an authorized manufacturer.
Traditionally, manufacturers solve this with barcodes or manual entry. Both are error-prone: barcodes get dirty or damaged, manual entry takes time and leads to typos. And neither helps much against counterfeit products.
The Solution
An RFID module in the device automatically recognizes consumables as soon as they’re inserted. No line of sight needed, no manual entry. The device instantly knows: What product is this? Is it genuine? Is it still valid? Does it match the current configuration?
For dialysis machines, this means: The filter is recognized, its usage time is stored on the tag, and the device warns when replacement is due. For infusion pumps: The medication set is identified, and the pump automatically configures itself to the correct parameters.
Why Manufacturers Want This
The obvious reason is patient safety – and the regulatory requirements that come with it. FDA 21 CFR Part 11 demands complete documentation, EU MDR requires traceability. With RFID, the device meets these requirements automatically.
The less obvious reason: aftermarket business. Consumables are often more profitable than the device itself. If the device only accepts original parts, it protects against cheap knockoffs and secures long-term revenue.
What We Deliver
We don’t build medical devices – we supply the RFID modules that device manufacturers integrate into their products. Compact form factors like the QR-NFC or RR15 fit even in portable equipment. The modules read and write tags, communicate via standard interfaces with the device controller, and are designed for long-term availability.
Medical devices have lifecycles of 10-15 years. Anyone integrating an RFID module today needs to be sure they can still get replacements in ten years. We can help you with that.
Developing a medical device with consumable recognition? Talk to our engineering team about integration.